Heart disease in the US (source, US Centers for Disease Control and Prevention)
Heart disease is the number one cause of death wordwide1. Early, accurate CVD risk assessment is part of established clinical care for primary prevention and disease management.
1. Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. [Accessed September 12, 2012]; Natl Vital Stat Rep. 2011 59:1–126. www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_10.pdf. [PubMed]
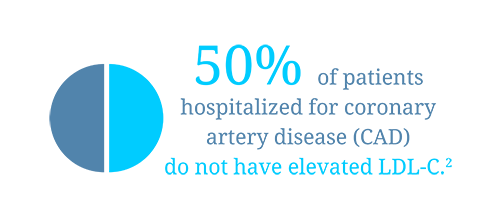
2. Sachdeva, A., et al., Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J, 2009. 157(1): 111-117 e2.
AXINON® lipoFIT®. |Innovative Lipoprotein Analysis.
Precision Diagnostics.

Our AXINON® lipoFIT® provides more precise information than the standard cholesterol test.
It measures concentration, size and distribution of the lipoprotein particles that carry cholesterol, as well as the quantity of triglycerides, cholesterol and phospholipids.
Available as CE-marked IVD in the EU
Technology. |The AXINON® System.
Easy. Fast. Efficient.

Nuclear magnetic resonance (NMR) spectroscopy is a proven and highly accurate test technology that assesses a variety of compounds at a molecular level.
Our AXINON® System, using FDA-cleared technology, is the core platform we use to develop diagnostic tests for illnesses stemming from metabolic dysfunction, including chronic heart, kidney and liver diseases.
This system incorporates diagnostic testing algorithms into nuclear magnetic resonance (NMR) spectroscopy for precise results.
To date, more than 3.5 million tests have been performed worldwide using Numares technology.